The Department of Pharmaceutical Sciences is a premier, research-oriented unit of the School of Pharmacy and Pharmaceutical Sciences dedicated to drug development and pharmaceuticals delivery to meet a wide array of healthcare needs. We are also committed to providing exceptional educational experiences for professional and graduate students.
The Pharmaceutical Sciences Department is housed in the top two floors of the University's new pharmacy school, in newly designed and constructed state-of-the-art laboratories and offices and houses about 20 faculty involved in teaching and research.
The department faculty work in collaborative groups covering many aspects of the drug development and pharmaceuticals delivery life cycle, including drug identification, pre-clinical testing, efficacy in mouse models, clinical trials and post-marketing research.
Message from the Chair
We are in an era of dramatic change in drug development, where there are many science-driven opportunities, but the systems to bring a lead compound to market are cumbersome and horribly expensive. As drugs become increasingly targeted, they also become increasingly personalized, with the extension that new drugs are likely orphan drugs for even common (stratified) diagnoses. This leads to a dilemma where there are few patients to recoup costs, and the dilemma of impressive opportunities that we cannot afford to develop and sell.
There has been little or no control on the costs of drugs in the U.S., and this has led to some questionable business models where old drugs are purchased by new biotech and the cost escalated several thousand fold. Perhaps better tolerated (and perhaps justified) are new orphan drugs, but costs at $300,000/patient/year are now not uncommon. Combining the above leads to an untenable, if not unethical, future for much of drug development.
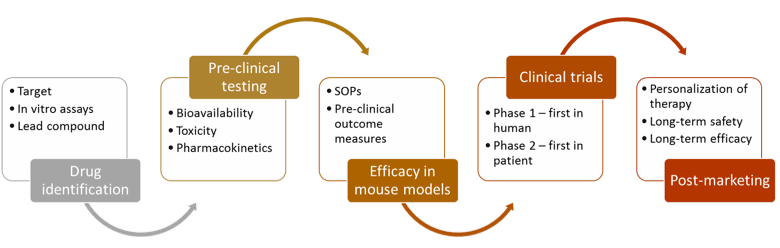
As the current system is unsustainable, it is clear that drug development paradigms need to change. Drug development needs to be faster and cheaper. How can this be accomplished? Better science to build a more compelling case for drug mechanism of action earlier in the process. Key in the new science of drug development will be pharmacodynamics biomarkers for dose selection and compelling surrogate outcome measures able to predict later improvements in quality of life.
Alternative business models are also needed, from focus on financial return (often seemingly independent of investment) to a shared risk-shared benefit model with stakeholders and governments; a model that ensures the greatest access to the most effective new medications. A key part of "shared risk" is "de-risking" – making more science-driven decisions on when to continue a program or pull the plug.
Who will drive the robust science that is needed to make the future of drug development sustainable? It seems the pharma industry is rapidly downsizing research groups worldwide. Instead, pharma is looking to universities and biotechs for new drugs to bring to market. So universities need to pick up the slack, but traditional universities can be slow to respond to changing technologies and business models, and methods of working with the private sector are often bogged down in months of red tape.
We feel that things can make sense if built from the ground up. Teams of interdisciplinary physicians and scientists can identify disease-specific targets, small molecules and antibodies can be quickly designed and tested directed to those targets, mouse models of human disease provide preclinical efficacy testing, and the time to NDA can be dramatically shortened via scientifically-robust use of biomarkers and smart design of clinical trials. Working hand-in-hand with industry works well if done in a smart way, with clear identification and management of inherent conflict of interest.
Goals
- Establish an internationally recognized pharmaceutical sciences research program with
a focus on development and implementation of emerging technologies in the drug development
pipeline. Focus topics:
- Biomarkers: Discovery, validation and qualification of biomarkers in drug development (pharmacodynamics; surrogate outcomes). Pharmacogenetics and pharmacogenomics will also be emphasized.
- Preclinical efficacy: Effective use of preclinical mouse models (genetic orthologs and avatars) for robust preclinical testing of promising therapeutic agents.
- Innovations in clinical trial design and conduct: Development and use of adaptive designs, inclusion of biomarkers as outcomes and use of mobile health for patient function in the community. Design and conduct of postmarketing trials for efficacy and quality of life in accelerated approvals will be a particular research strength.
- Establish synergistic interactions with both stakeholder groups (nonprofits) and industry (for-profits) on a global scale.
- Form industrial collaborations and contracts for biomarker discovery, validation and qualification using the department cores.
- Train the next generation of pharmacists and pharmaceutical research scientists, well-versed in emerging key areas of drug development, prescription and patient monitoring (preclinical science, clinical trial science, pharmacogenomics, poly-pharmacy, regulatory science and biomarkers).
Transdisciplinary Areas of Excellence (TAEs)
Binghamton University's leadership and faculty carried out a process of defining transacademic areas of research and education focus, called Transdisciplinary Areas of Excellence (TAEs). Six TAEs were defined: citizenship, rights and cultural belonging; data science; health sciences; material and visual worlds; smart energy; and sustainable communities.
The School of Pharmacy and Pharmaceutical Sciences was designed and founded to directly address the Health Sciences TAE, and research and education programs will be built around these University-wide themes.
- Vision: The Health Sciences Initiative at Binghamton University will improve health through cutting-edge basic, applied and translational research in health science fields and healthcare systems.
- Theme 1: Disease Susceptibility, Pathogenesis and Prevention
- Pharmaceutical sciences: brings existing projects and broad experience
- Exemplar: Leading study of 1,000 school children in two-year interventional and molecular marker study of pro-inflammatory state
- Theme 2: Sensors and Devices for Diagnosis and Treatment
- Pharmaceutical sciences: molecular diagnostics and biomarkers expertise
- Exemplar: Integration of 1,200 protein aptamer panels into PK and PD studies, clinical trials
- Theme 3: Healthcare Systems and Outcomes Research
- Regulatory expertise in both FDA and EU; focus on bringing down drug costs
- Exemplar: Venture philanthropy model to de-risk and reduce cost
- Theme 4: Individualized Therapeutics
- Expertise in pharmacogenetics, polypharmacy monitored by biomarkers
- Exemplar: Pharmacists and the rapidly growing postmarketing space
Sincerely,
Aaron Beedle
Associate Professor and Chair
Department of Pharmaceutical Sciences