Malaria and Drug Resistance
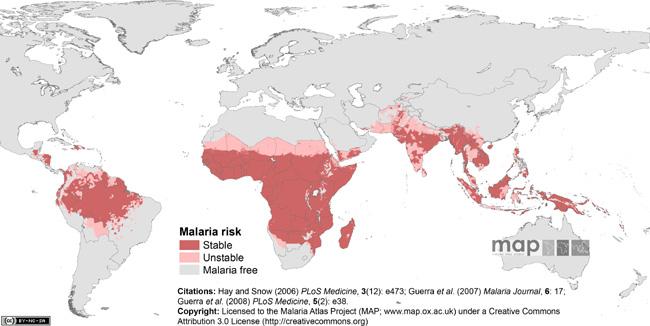
Malaria affects almost half of the world's population today. Annually, it is responsible for up to 500 million clinical cases and between one to three million deaths, mostly in children under the age of five. Global malaria eradication and control programs rely on multi-faceted strategies that include residual indoor spraying with insecticides to control Anopheles vectors, use of bed nets to reduce transmission, and administration of antimalarials for both prophylaxis and active treatments. Binghamton University's Biomedical Anthropology Program faculty members J. Koji Lum and Ralph M. Garruto, and students Chim Chan, Dana Reiff and Rita Spathis are examining how cloroquine resistance has developed in isolated Pacific populations with endemic and epidemic disease.
Historically, chloroquine had been the antimalarial of choice because it was inexpensive, effective against all four Plasmodium species that infect humans, and well tolerated even among children and pregnant women. Chloroquine in its active form targets the digestive vacuole of the parasite and interferes with the elimination of the toxic heme. After invading the red blood cells, the parasite breaks down hemoglobin in the digestive vacuole for essential amino acids and polymerizes the iron-containing heme to nontoxic hemozoin. Chloroquine prevents such polymerization by binding to hemozoin, resulting in the buildup of toxic heme that disrupts normal membrane functions and kills the parasite.
The widespread use of chloroquine as a cure and a prophylaxis for P. falciparum, the most debilitating of the four human malaria species, contributed to the rapid appearance of chloroquine resistance. Cases of chloroquine treatment failure were first documented independently on the Thai-Cambodian border and in Peru in the early 1960s. From these two initial foci, chloroquine resistant P. falciparum rapidly spread to other regions within the next two decades. By 1980, chloroquine resistance was rampant and necessitated the replacement of chloroquine by other drugs such as sulfadoxine-pyrimethamine as the first-line malaria treatment in many countries.
Recent genetic studies have identified Papua New Guinea as another independent origin of chloroquine resistant P. falciparum. Using archived human sera collected at various locations in the Pacific over a 40-year period (1950 to 1990), the research team initiated a study to examine the evolution of chloroquine resistance in the Pacific, with three related objectives, (1) to test the hypothesis that genetic polymorphisms associated with chloroquine resistance accumulated in a stepwise fashion over time, (2) to address the potential invasion of chloroquine resistant P. falciparum in the Pacific using parasite mitochondrial DNA, and (3) to assess the strength of chloroquine selection using microsatellite variation.
Increasing incidence of treatment failures with other antimalarials warrants the reexamination of chloroquine as a viable alternative. The researchers believe that elucidating the genetic basis of chloroquine resistance, combined with improved techniques for monitoring and case management, will prolong the efficacy of chloroquine and other antimalarials and contribute to the public health policies and malaria control.